我们在您的浏览器上使用 cookie 来定制内容以供您查看和分析。如果您点击“接受”或继续浏览我们的网站,我们假设您已经同意我们使用 cookie。有关详细信息,请参阅我们的 Cookie 政策。
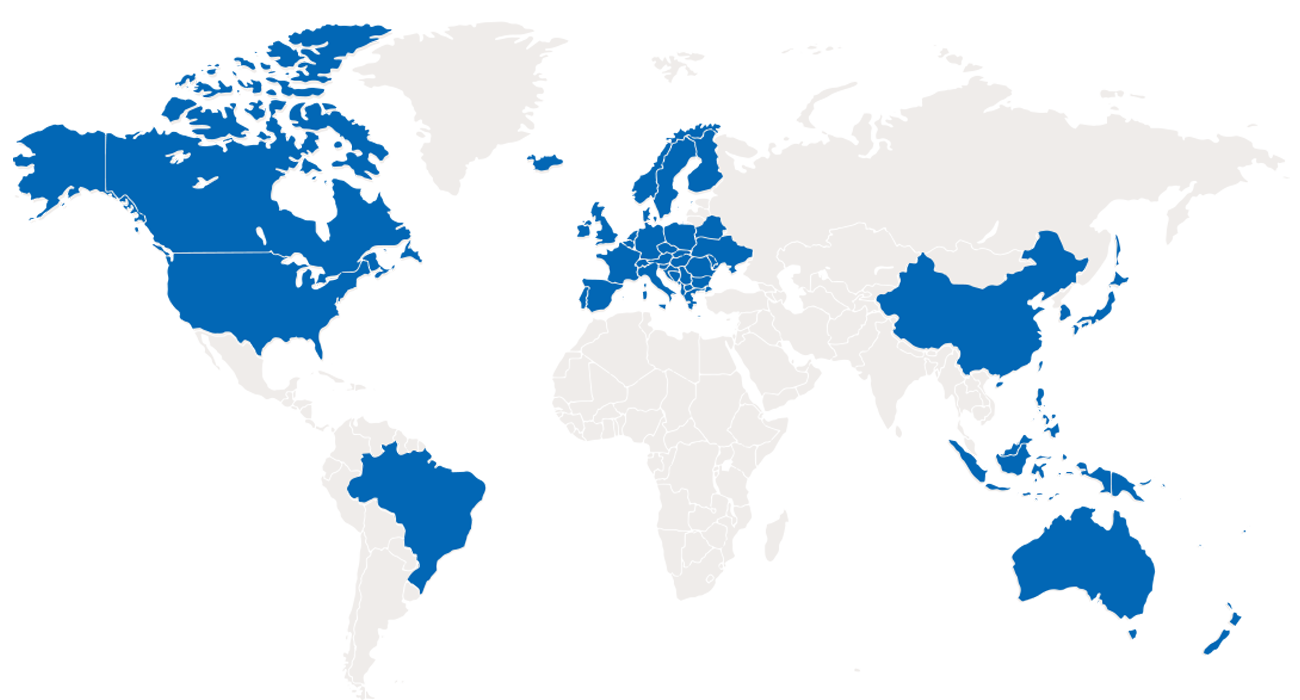








帕母医疗始终以临床价值为驱动,致力于为肺高压以及心衰领域提供突破性疗法,提升患者的生命复原力。作为写入国际指南的中国原创器械领航者,帕母医疗凭借其深厚的临床资源,专注于突破性技术的科学探索及国际视野的商业化开拓,力图在肺高压以及心衰不同阶段创新更优的解决方案,以期延缓和改善患者的疾病进展,以科技创新力量构建肺高压及心衰行业治疗新格局。
12月 PADN导管成功进入NMPA创新医疗器械特别审查程序流程
1月 PADN-CFDA注册临床研究完成了第一例患者入组
12月 PADN首次被写入《中国肺高血压诊断和治疗指南2018》
2月 PADN-5临床研究结果发表在JACC: Cardiovascular Interventions杂志
8月 完成A轮融资
8月 PADN首次录入欧洲ESC肺高压指南
9月 PADN-CFDA注册临床研究结果在美国经导管心血管治疗学术会议(TCT 2022)盛大公布
10月 完成B轮融资
3月 PADN-5 3年临床研究结果在第二届心力衰竭治疗与器械会议(THT 2023)盛大公布
8月 PADN-PH-HF中国注册临床研究完成国内多地临床中心启动及患者入组
12月 PADN获美国FDA人道主义用途器械(HUD)认定
12月 PADN系列产品获得国家药监局批准上市
3月 上市后全球首例PADN手术在厦门大学附属心血管病医院顺利开展
6月 PADN技术被写入《中华心血管杂志》指南与共识
6月 PADN-COLUMBUS Trial(治疗II型肺高压)在葡萄牙完成启动及首批患者入组
11月 PFlexi鞘管获得FDA上市批准
1月 PFlexi鞘管获得中国国家药监局(NMPA)批准上市
3月 完成近1亿美元C轮融资
3月 PHD360肺动脉射频消融仪正式被中国国家药品监督管理局(NPMA)批准上市
4月 PADN系列产品获得美国FDA临床试验器械豁免(HDE-IDE)批准,用于开展一型肺动脉高压患者(Group 1)的临床试验
5月 PADN系列产品成功通过MDR认证并获得欧洲上市许可
5月 完成数千万美金C+轮融资
6月 PADN系列产品在阿联酋成功完成首三例海外商业化手术
8月 PADN被写入《中国循环杂志》指南与共识
9月 PADN系统获得美国FDA临床试验豁免(PMA-IDE)批准,用于开展二型肺高压(Group II PH)患者的临床研究。还获得美国医保局(CMS)批准取得产品临床阶段、上市后美国医保全覆盖












60
7000
7000
5
5
100










技术了解、企业合作请联系
pr@pulnovomed.com
021-63660305